Molar mass of He = 4.002602 g/mol
Helium weighs 0.0001785 gram per cubic centimeter or 0.1785 kilogram per cubic meter, i.e. Density of helium is equal to 0.1785 kg/m³; at 0°C (32°F or 273.15K) at standard atmospheric pressure. In Imperial or US customary measurement system, the density is equal to 0.01114 pound per cubic foot lb/ft³, or 0.00010318 ounce per cubic inch. The Helium Consensus Protocol is outlined in detail in Section 6. 2.3Physical Implementation The Helium network is also a physical wireless network instantiation. The participants in the Helium network can be thought of as follows: WHIP The Helium network uses a new open wireless pro-tocol, called WHIP. WHIP is a long-range, low-power. The Ultrastar Helium Platform (He 6) hard drive family is HGST’s latest effort to provide the next step up in capacity for enterprises that are faced with ballooning data volumes. Exclusive Helium Merch including Tees, Hoodies, Stickers and Beanies. Get Helium Updates to your Inbox. Company Home Ecosystem About Us Console Support Careers Brand Guidelines Blog Whitepaper. Get Involved Mine Stake Use Buy your Hotspot Developers Host Guide.
Convert grams Helium to moles or moles Helium to grams

| Symbol | # of Atoms | Helium | He | 4.002602 | 1 | 100.000% |
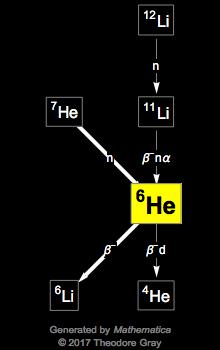
Helium 6
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass.
Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. We use the most common isotopes. This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass.
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.
Helium 6.0
Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.
Helium 600
Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights.

